Symbol

Title
Manufacturer
Standard*
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Description
Indicates the medical device manufacturer.

Authorized representative in the European Community/European Union
ISO 15223-1 Medical devices – Symbols to be used with medical device labels – General requirements
Indicates the Authorized representative in the European Community/European Union.

Date of Manufacture
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the date when the medical device was manufactured.
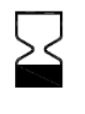
Use-By Date
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the date after which the medical device is not to be used.

Batch Code
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the manufacturer’s batch code so that the batch or lot can be identified.

Catalogue Number
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the manufacturer’s catalogue number so that the medical device can be identified.

Serial Number
ISO-15523-1 Graphical symbols for use on equipment – Registered
Indicates the manufacturer’s serial number so that a specific medical device can be identified.

Non-Sterile
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates a medical device that has not been subjected to a sterilization process.

Do not use if package is damaged and consult instructions for use
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates that the medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions use for additional information.

Fragile, handle with care
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates a medical device that can be broken or damaged if not handled correctly.
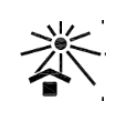
Keep away from sunlight
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates a medical device that needs protection from heat and radioactive sources.

Keep dry
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates a medical device that needs to be protected from moisture.

Lower limit of temperature
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the lower limit of temperature to which the medical device can be safely exposed.

Upper limit of temperature
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the upper limit of temperature to which the medical device can be safely exposed.
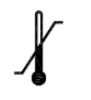
Temperature limit
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the temperature limits to which the medical device can be safely exposed.

Do not re-use
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates a medical device that is intended for single use only.

Consult instructions for use or consult electronic instructions for use
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the need for the user to consult the instructions for use.

Caution
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or the current situation needs operator awareness or operators’ action in order to avoid undesirable consequences.

Alternating Current
IEC 60417 Graphical symbols for use on equipment
To indicate on the rating plate that the equipment is suitable for alternating current only; to identify relevant terminals.
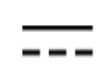
Direct current
IEC 60417 Graphical symbols for use on equipment
To indicate on the rating plate that the equipment is suitable for direct current only; to identify relevant terminals.
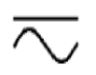
Both direct and alternating current
IEC 60417 Graphical symbols for use on equipment
To indicate on the rating plate that the equipment is suitable for both direct and alternating current (universal); to identify relevant terminals.

Class II equipment
IEC 60417 Graphical symbols for use on equipment
To identify equipment meeting the safety requirements specified for Class II equipment according to IEC 61140.

Type BF applied part
IEC 60417 Graphical symbols for use on equipment
To identify a type BF applied part complying with IEC 60601-1.

General Warning Sign
ISO 7010 Graphical symbols – Safety colours and safety signs – Registered safety signs
To signify a general warning.
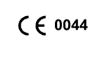
TUV Notified Body Number
This Product is in conformity with European Medical Directive 93/42 EEC. This has been verified by a notified body.
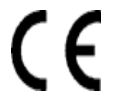
Conformité Européene or European Conformity
Council Directive 93/42/EEC of 14 June 1993 concerning medical devices
Indicates manufacturer declaration that the product complies with the essential requirements of the relevant European health, safety and environmental protection legislation.

Symbols to be used with medical device labels, labeling, and information to be supplied
ISO 15223-1
Indicates the item is a medical device.
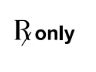
Prescription Use Only
Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner.

Warning; Laser beam
ISO 7010
To warn of a laser beam.

Wireless signal level, very strong signal
IEC 60417
To indicate that the wireless signal level is very high.

For indoor use only
IEC 60417
To identify electrical equipment designed primarily for indoor use.

Wear eye protection
ISO 7010
To signify that eye protection must be used.

Wear protective gloves
ISO 7010
To signify that protective gloves must be worn.

Collect separately
DIRECTIVE 2012/19/ EU
Separate collection for waste of electrical and electronic equipment. Do not dispose of battery in municipal waste. The symbol indicates separate collection for battery is required.

Federal Communications Commission
21 CFR Part 15
Meets FCC requirements per 21 CFR Part 15.

ETL Listed Mark
The ETL Mark is proof of product compliance to North American safety standards. DenMat’s ETL Mark comes from Intertek Testing Services, Inc.

Unique device identifier
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates a carrier that contains unique device Identifier information

Internal Symbol
Do not use in the presence of flammable substances.
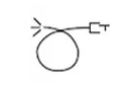
Laser aperture at end of handpiece
Internal Symbol

Caution: Do not wind clockwise
Internal Symbol

Caution: risk of electric shock
IEC 60417 — Graphical Symbols for Use on Equipment
To identify equipment, for example, the welding power source, that has risk of electric shock.

Warning: Hot surface
ISO 7010 — Graphical symbols — Safety colours and safety signs — Registered safety signs
To warn of a hot surface.
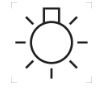
Lamp; lighting; illumination
IEC 60417 — Graphical Symbols for Use on Equipment
To identify switches which control light sources, e.g. room lighting, lamp of a film projector, dial illumination of a device.
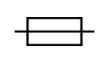
Fuse
IEC 60417 — Graphical Symbols for Use on Equipment
To identify fuse boxes or their location.
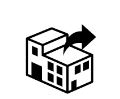
Distributor
ISO 15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer — Part 1: General requirement
To indicate the entity distributing the medical device into the locale.

Li-Ion Battery
Internal Symbol
To indicate that a Lithium battery must be recycled.

Authorized representative in Switzerland
Indicates the Authorized representative in Switzerland.
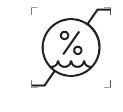
Humidity limitation
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the range of humidity to which the medical device can be safely exposed.
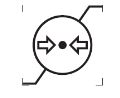
Atmospheric pressure limitation
ISO-15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Indicates the range of atmospheric pressure to which the medical device can be safely exposed.
*All standards listed are assumed to be the latest revision unless otherwise specified.
